Abstract
Background: CT-P10 is the first biosimilar to the innovator rituximab (RTX), which was approved by the European Medicines Agency for all indications for which RTX is approved. Pharmacokinetic equivalence and comparable efficacy was demonstrated in advanced-stage follicular lymphoma patients (Kim WS et al. Lancet Haematol. 2017; S2352-3026(17)) as well as in rheumatoid arthritis (Yoo DH, et al. Arthritis Rheumatol. 2016; 68 (suppl 10)). It is known that there are several factors affecting on pharmacokinetics (PK) of rituximab. However, there are limited data on comparing PK data between a biosimilar of rituximab and RTX with different subgroups in advanced-stage follicular lymphoma (AFL) patients.
Objective: This report is to investigate the PK of CT-P10 according to the several relevant clinical factors and to compare with RTX in the post-hoc analysis of randomized controlled trial to compare CT-P10 and RTX in advanced-stage follicular lymphoma (NCT02162771).
Methods: A total of 121 patients of PK population were included in this analysis. Fifty nine patients in the CT-P10 group and 62 patients in the RTX group received CT-P10 or RTX (375 mg/m2 i.v) plus CVP (cyclophosphamide, vincristine and prednisone) for every 3 weeks over 8 cycles. Area Under the Concentration-time curve at steady state (AUCtau) and the maximum serum concentration at steady state (CmaxSS) at Cycle 4 and trough serum concentration (Ctrough) at Cycle 4 and Cycle 8 were calculated by standard non-compartmental methods using Phoenix WinNonlin. The parameters were analysed by subgroups of Gender (male vs. female), Eastern Cooperative Oncology Group (ECOG) Performance Status (0 vs. ≥1), Follicular Lymphoma International Prognostic Index (FLIPI) Score (0-2 vs. 3-5) and its component (Age (≥60 vs. <60), Hemoglobin [Hb] (≥120g/L vs. <120g/L), Lactate dehydrogenase [LDH] (>UNL vs. ≤UNL), number of nodal sites (>4 vs. ≤4)), presence of bulky lesion at baseline (≥7cm vs. <7cm) and Complete response (CR)/Complete response unconfirmed (CRu) as best overall response assessed by International Working Group 1999 in the each and combined group of CT-P10 and RTX. Outliers which determined by robust regression outlier testing were excluded in this analysis. For AUCtau, 7 patients (5 in the CT-P10 group and 2 in the RTX group) and among them, 4 patients (2 patients in each group) for CmaxSS were defined as outliers.
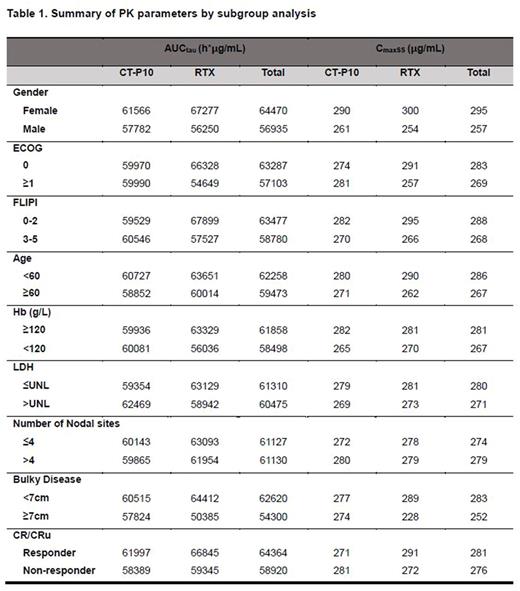
Result: Higher AUCtau and CmaxSS were observed in patients who are female, under 60 years old, good ECOG Performance Status, low FLIPI score, normal Hb and LDH levels, and non-bulky disease at baseline as shown in Table 1. In patients who achieved CR/CRu as their best overall response during 8 cycles showed higher AUCtau, CmaxSS and Ctrough than in patients who did not achieve CR/CRu response in both CT-P10 and RTX groups. At Cycle 4 and Cycle 8, mean Ctrough in the CR/CRu responder were 72 μg/mL, 108 μg/mL, respectively, and those concentrations are 56 μg/mL, 96 μg/mL in the CR/CRu non-responder. Importantly, there was no statistically significant difference between the CT-P10 and RTX in any of subgroup analyses; confidence interval of the ratio of geometric mean of CT-P10 to RTX contains 1 using Satterthwaite approximation for each comparison.
Conclusion: Female gender, younger age (<60), better ECOG Performance Status, lower FLIPI score, LDH and Hb levels in normal range, absence of bulky disease at baseline and better response are factors associated with increased serum rituximab concentration in the combined treatment groups. No statistically significant differences were found between CT-P10 and RTX groups in PK at all subgroup analyses. Therefore, this result shows that the PK of CT-P10 is in accordance with the RTX from historical data and further supports the PK similarity between CT-P10 and RTX.
Kim: Celltrion, Inc: Consultancy, Honoraria; Roche: Research Funding; Mundipharma: Research Funding; Novartis: Research Funding; Kyowa-Kirin: Research Funding; Donga: Research Funding; J&J: Research Funding; Takeda: Research Funding. Coiffier: Celltrion, Inc: Consultancy, Honoraria. Buske: Hexal: Honoraria; Celltrion, Inc.: Consultancy, Honoraria; Pfizer: Honoraria; Gilead: Honoraria; Janssen: Honoraria, Research Funding; Roche: Honoraria, Research Funding. Ogura: SymBio: Consultancy, Research Funding; Celgene: Consultancy, Honoraria; Astrazeneca: Honoraria; Takeda: Consultancy, Honoraria; Mundipharma: Consultancy; MeijiSeika Pharma: Consultancy; Celltrion, Inc: Consultancy, Honoraria. Kwak: Celltrion, Inc: Consultancy; InnoLifes: Consultancy, Equity Ownership; Pepromene Bio: Consultancy, Equity Ownership. Lee: Celltrion, Inc: Employment. Bae: Celltrion, Inc: Employment. Kim: Celltrion, Inc: Employment. Kim: Celltrion, Inc: Employment. Lee: Celltrion, Inc: Employment. Kwak: Celltrion, Inc: Employment. Lee: Celltrion, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal